- Super User
- Progress
- Read Time: 1 min
- Hits: 0
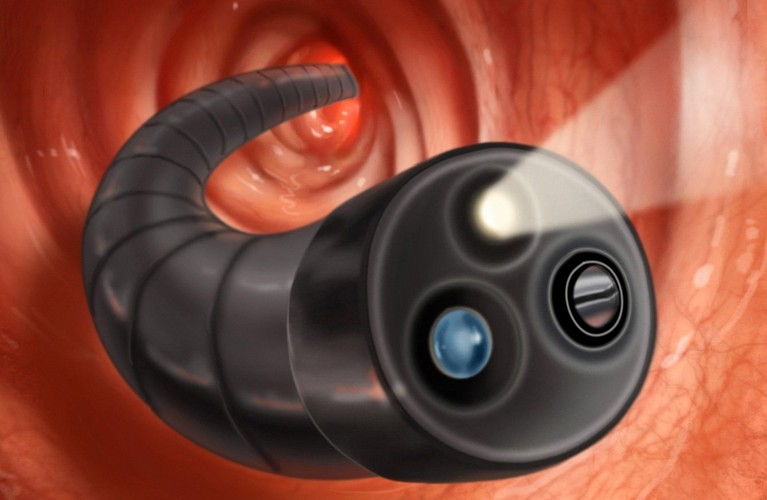
Permission was obtained from the Ministry of Health of the Russian Federation to conduct three Phase III clinical trials to assess the efficacy and safety of a new oral medicine for patients with moderate or severe Crohn's disease, sponsored by the pharmaceutical company Selgene, Switzerland.