- Super User
- Trials
- Read Time: 1 min
- Hits: 0
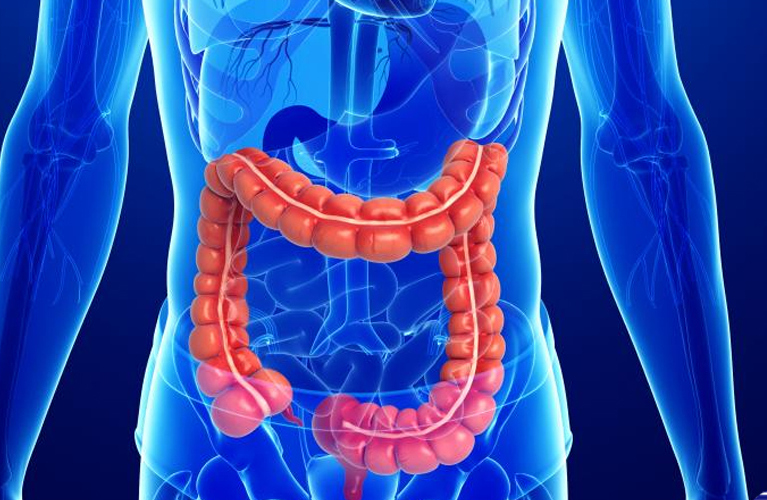
Another visit of the opening of the center was conducted: we study the drugs of the pharmaceutical company Eli Lilly in a randomized, double-blind, placebo-controlled phase II trial with adaptive design in patients with moderate and severe ulcerative colitis.